Want to Change Your DNA?
Exploring New Paths on an Evolving Genetic Landscape
Biochemist Jennifer Doudna discusses cutting-edge DNA technology that enables the editing of the human genome. It also adds to a growing list of current ethical issues faced by researchers.
Growing up on the Big Island of Hawaii, Jennifer Doudna was accustomed to the great curve of the horizon and the vast sky that envelops this tiny mid-ocean vantage point. It’s a vista that can be intimidating in scale yet inviting in possibility: you’re practically lost in space, yet everywhere is just there, over the edge, below the surface, at your feet. This is an apt picture for the discovery for which Doudna is best known. Inside the tiniest of bacteria cells she and her team discovered a gateway to a whole new world: within the chemistry whereby bacteria can remember and cut up DNA delivered by infectious viruses, she found a tool for editing genes.
Although DNA-cutting molecules (called restriction enzymes) had been known for decades, this was something new—an enzyme that could be programmed where to cut. Almost out of nowhere, this breakthrough has slung open a whole new window into genetic engineering at a level of precision and accuracy unattainable before.
Doudna and her research partners suggested in their 2012 paper in Science, “We propose an alternative methodology based on RNA-programmed Cas9 that could offer considerable potential for gene-targeting and genome-editing applications.”
Known as CRISPR-Cas9 (or simply “crisper”), it has indeed taken the genetic engineering world by storm. As a recent review paper notes, “CRISPR has quickly become the preferred tool for genetic manipulation, and shows incredible promise as a platform for studying gene function in vivo.”
“I am excited about the potential for genome engineering to have a positive impact on human life, and on our basic understanding of biological systems,” Doudna says. But what is the best path forward into this new world?
“Engendering more trust in science is best achieved by encouraging the people involved in the genesis of a technology to actively participate in discussions about its uses. This is especially important in a world where science is global, where materials and reagents are distributed by central suppliers and where it is easier than ever to access published data.”
Doudna is a professor of biochemistry, biophysics and structural biology at the University of California–Berkeley. She spoke with Vision contributor Dan Cloer in her office on the Berkeley campus.
DC What is at issue when we talk about germ-line editing? How did the December 2015 International Summit on Human Gene Editing affect your views?
JD This is such a fantastical thing to think about. You suddenly realize, “Wow, we have a tool that in principle allows us to change human evolution. We can wipe out a mutation from the whole population—just get rid of it.”
There are scientific practicalities and human reproduction realities, of course, so we are not going to do anything overnight. But the big-picture view is that we have the tools to change our DNA and change the things that we are passing on to future generations. And now we can make those decisions. That is a profound thought.
“We have the tools to change our DNA and change the things that we are passing on to future generations. And now we can make those decisions.”
The recent meeting did not change these views, but it broadened them. I’m a pretty open-minded person, and I’m trying to learn and understand. I know the science because I’m a scientist, but I’m trying to understand all the other points of view about this. The summit was interesting because we heard from a number of bioethicists and people working with patients and families with genetic disorders. I have interacted with people who have these diseases, with desperate parents wanting help. I am a parent, so I can totally understand their desires.
The summit achieved an important goal, which was to bring key stakeholders together; not only scientists but people from other areas of life—not everyone, but it was a start. So a number of views were expressed, and that was great. In general there was agreement that research should move ahead, but we want to proceed with appropriate caution. This is why our statement at the end of the meeting aimed for broad societal consensus before we use this technology in any clinical application in the human germ line. But how do you define “broad societal consensus”? That remains to be seen; it was not the end of the conversation but the beginning.
DC At the end of the summit there was discussion about the language, that a glossary would be needed for lay observers to access this information. I had the sense that the “luminaries” (as David Baltimore referred to the speakers and audience) were the adults in the room, and the rest of us were children who needed to be taught or led to some conclusion. Can the public understand what it needs to know?
JD I feel a little bit sad about that question; I certainly do not feel that way and am sorry for that impression. We are all people living in the same society and planet together. Yes, some of us have expertise in science and others in something else, but we are all relevant to this discussion. I am every bit a student in those other areas, just as they might be students of science. I want to participate in the conversation, not dictate or tell other people what to think. However, the reality is that scientists do have a certain base of knowledge.
The challenge is how to communicate that information to people who are not technical experts. Science is very much a language, and we get attached to our acronyms. I feel that it’s critical that scientists are involved in this conversation, but we need everybody else at the table too. I want people to think about these things from an educated position—to understand the science well enough that they can decide how they really feel about it. I just want to be sure that, as a scientist involved in this, I’m doing my part to provide education—to explain what this is so that people can think about it from an informed point of view. I don’t want to tell them how to think; I just want to tell them, “This is what’s going on. Now you decide.”
DC In an article in Nature you wrote, “The rapid development and widespread adoption of easy-to-use, inexpensive and effective genome-editing methodologies has changed the landscape of biology.” Given that for the lay person this is extremely complex, can you tell us what, in essence, it means?
JD It means that now we have a tool that allows rewriting of the genetic code, changing the DNA in cells. That’s a profound thing. It allows scientists to do things that in the past would have been really hard or impossible. When I think of the “landscape of biology,” I’m thinking of everything; it’s not only human biology and medicine but also plant biology, fungi, the bacterial world. It’s a big place.
DC Is there also a dark area here? How easy would it be to engineer a superbug?
“If people are worried about bioterrorism, there are far easier ways to cause problems than this.”
JD It could be done with difficulty; but if a terrorist is looking to cause harm, there are much easier ways to go. That’s not something I worry too much about. When we say this is “a simple and easy tool,” we’re speaking relatively. If you’re a molecular biologist, it’s truly simpler and easier, but for the average person on the street that’s not true. If people are worried about bioterrorism, there are far easier ways to cause problems than this.
DC Fifty years ago the term algeny was coined from alchemy and gene, reflecting some skepticism about what we could actually accomplish by altering genetics. Are we overly enamored with genes? Where are we in understanding their deterministic strength?
JD This is going to be a moving target. Today we know a set of genes that cause specific monogenic disorders [i.e., stemming from a single defective gene: Tay-Sachs disease, sickle cell anemia, cystic fibrosis, hemophilia, etc.]. And if we were able to fix or repair those mutations in patients, we know that would have profound benefits for those patients. Going beyond that to much more complex diseases, like schizophrenia, autism spectrum disorder or others where many genes are involved (maybe a hundred genes or more, where each may contribute a little bit) will be difficult. How would you ever think about using a genome-editing approach to those? I don’t know. Would gene editing ever be appropriate? We are certainly not there today, and we may never be there.
There are all sorts of other things where we actually have no idea what the underlying genetic basis is. But I do think that more knowledge will become available in the future. I have no specific timeline, but I believe the genome-editing technologies will help in that basic understanding. We will be able to dissect the genetics of other traits and diseases as we go forward. But as of today, if someone really wanted to have that taller, smarter child with this level of intelligence, it’s not as if we could dial that into the genome.
DC That kind of enhancement creates another set of questions, but for monogenic disease the questions are seemingly simpler and could be addressed.
JD All the problems are not solved, for sure. But if this is your problem, then this is huge. My research is about the gene-editing technology and also bacterial immune systems. We collaborate with clinicians concerning using the technology to try to understand the genetic basis for certain diseases, and we hope that in the future there will actually be a benefit for patients. I am not a medical doctor, so I’m not doing clinical things myself; but I do want to enable those who do. These are all adult-somatic-cell treatments, not germ-line.
“All the problems are not solved, for sure. But if this is your problem, then this is huge.”
I do want to be involved in the discussion across the board on this; I am not going to just lob this over the fence and let people “over there” sort it out. It is fair to say I am part of that conversation and I feel a sense of responsibility.
DC What was your contribution to the CRISPR-Cas9 discovery? How does gene editing work?
JD The CRISPR work was a collaboration between me and Emmanuelle Charpentier in Germany. We teamed up to figure out the molecular function of a particular protein called Cas9, which works in the bacterial immune system. Our research uncovered the fact that this was an RNA-guided protein.
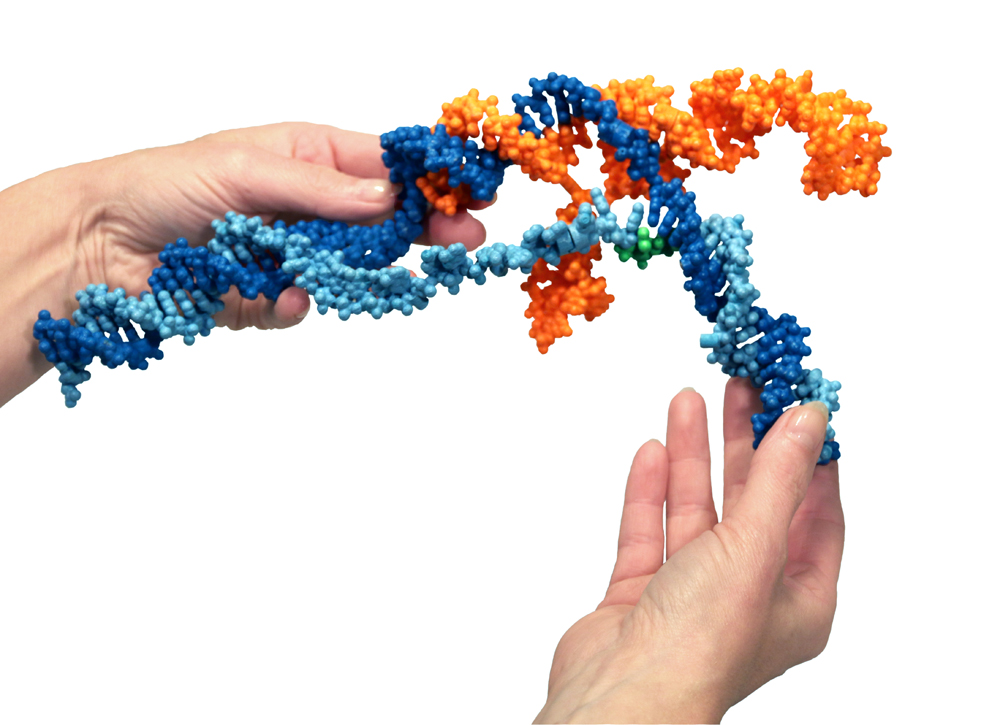
This plaster and nylon custom model of the CRISPR-Cas9 System was designed and printed by 3D Molecular Designs in collaboration with Jacob Corn, PhD, scientific director of the Innovative Genomics Initiative.
Photo: Shannon Smith, 3D Molecular Designs
Here’s a model. The protein binds with an RNA molecule [orange]. The RNA sits inside the protein. Together they run along the DNA [blue] of the cell searching for a match between the genetic letters in the DNA with the letters of the RNA. That’s how the RNA is a “guide” in the system; it looks for a specific DNA sequence. When a match is found between the 20-letter sequence of the RNA and a segment of the DNA, the DNA double helix is opened (the light blue strand is separated from the dark blue and an RNA-DNA helix is formed, tucked away inside the protein). Then molecular “blades” in the protein cut the two DNA strands. This makes a very precise cut, like with a scalpel; the DNA ladder is sliced straight across. For bacteria, this is a way to counter a viral infection.
Other machinery in the cell repairs the break. When the repair occurs, the actual editing happens to the DNA and the sequence can be changed. Cas9 is the scissors, but then the cut DNA must be handed off to other proteins in the cell that catalyze recombination. These repair enzymes can find DNA to stitch in, which can be supplied by the experimenter.
These repair molecules have been known for a long time. So the idea was that if you could figure out a way to break DNA at a particular place where you want to induce a change, you could control the way the repair happens. That was the challenge—how to make the cut where you want it.
Even when I was in graduate school in the 1980s people were thinking about how to do this. I was at the Massachusetts General Hospital when Jim Gusella mapped the mutation that causes Huntington’s, a fatal neurological disease. And everyone was thinking, So now we know the gene, but how do we fix it? There were all kinds of ideas: What if you had a chemical that could break it? Or use another piece of DNA to break it?
When we came across this protein in the bacterial immune system, we weren’t looking for that, of course; but once we understood how it worked we realized this would be an incredible tool for genome editing. Because we can program it with this little piece of RNA, we can make a break wherever we want to.
“Once we understood how it worked we realized this would be an incredible tool for genome editing.”
DC How do you get it to the right place, the right cells?
JD Ah yes, this is a very important challenge going forward, especially for therapies. Right now the way we do this is kind of crude: We can electrically stimulate the cells or treat them chemically to take up the molecules. We can hook it up to an antibody which is then recognized by specific molecules on certain cells. But we don’t honestly have a great way right now to introduce this into, say, muscle or brain or liver or lung cells.
So in terms of using this as a therapy, a delivery system is at the leading edge of the field. I am extremely interested in this and am working with various colleagues to try to figure it out. I do think the first human therapies using this technology will be with blood or the eye, because delivery into these two tissues does not seem to be such a problem. In the eye you can inject material, and with blood you can remove the cells from the body and treat them outside. Then you can validate that the editing was done correctly, grow up the cells, and reintroduce them to the patient.
In those two situations the delivery challenge is not so great, but in other problems, like treating Duchenne muscular dystrophy for example, there are still many things to figure out. Using engineered viruses that can locate and attach to the right cells is another method, but there are risks that need to be overcome if this is to be used in humans.
We know that in principle this can work in vitro—changing cells outside the body—but it’s important to appreciate that this has already been used to treat and cure disease in animals. In mice, for example, we know that this works, but to move it to humans and use it safely is a big step.
[Since the time of this interview several labs have reported using a viral delivery system to correct the Duchenne mutation in mice.]
DC Will the technology become so accurate that current GMO issues will be put aside?
JD Yes, it’s coming. Not yet, but the technology is coming. This is a really precise tool. Instead of requiring a virus to integrate randomly into a genome and deliver a new gene (how the original gene therapy was done, for example), now we are programming an enzyme to go to a specific place in a cell’s DNA and make one or more cuts. Then, very precisely, a change or a replacement of the information can be made. Soon we will be able to do that with extreme accuracy and incredible efficiency. This is why everybody is so excited. It is a profound change in how we think about and do biology. That is part of the new landscape.
DC Cas9 is a protein so there must be a gene for it. Bacteria have that gene. Do we have it too?
JD No, not as far as we know. Eukaryotic cells [cells with a nucleus that houses the DNA] don’t seem to have it, or we just haven’t found it yet. But if they don’t, why not? Maybe it’s because our cells have much more sophisticated immune systems. Our system of innate immunity fights viruses through a series of proteins, and our adaptive system creates antibodies, so maybe we don’t need something like this.
DC This is true in a multicellular creature, but all our problems begin as single cells. Cancer begins as a single cell, so if there was a repair mechanism—
JD Yes, but the cell would have to know that it has a mutation that’s bad and then be able to fix it. The “knowing” would be the tricky part. But stay tuned!
DC Have you been surprised by the depth of activities going on inside the cell?
JD I am surprised every day. That’s one of the joys of being a scientist. We humans are fascinating creatures, and the whole way that cells grow and divide and are organized—I never know what to expect next. Who knew Cas9 existed and did these things? Science is a process like peeling the onion: take away one layer and then you realize, “Oh, there are five more under there.” Layer upon layer of controls just in the genome, for example. It’s not just about genes encoding protein, right? It’s about how those genes are regulated, and all the chemical modifications that are made to the DNA, and the DNA’s mobility and plasticity. That is all extremely interesting.
DC As you imagine the future of gene editing, is anything scientifically or ethically out of bounds, where the costs are too high for the benefits?
JD I am a scientist and I believe that it’s important to have data to make decisions, especially about things like this. But I do think that experiments—particularly with humans, animals or human embryos—must be done with appropriate guidelines in place to avoid abuse. It is part of our human nature to want to have regulations or consensus agreements in place so that people proceed in a way that is comfortable for society. What is challenging, however, is that science is global, and different societies and cultures have different norms for how they think about these questions. So I don’t know that we’re going to get to a point where everybody on the planet can say we all agree that we can do X and Y but not A and B.
“Science is global, and different societies and cultures have different norms for how they think about these questions.”
But I do think that within some jurisdictions, and probably within the mainstream scientific and clinical communities (in the United States and Europe at least, and maybe in Asia too) there will be consensus about how to proceed in a way that is respectful of human life and where most people would feel comfortable about moving forward.
Change always takes time. You and I are of the same vintage, so we’re old enough to remember when in vitro fertilization (IVF) first became widely available. There was a lot of dispute about whether or not this was okay to do; I remember debates in my family—my parents wondering, “Is that right? Should people really be able to do that?” But over time people become comfortable with it; they found that babies born this way were healthy, and they made their parents very, very happy. These children brought great joy. Is that a bad thing? Some people might say, “I would never want to do that,” but others would say IVF is a wonderful thing.
Will we ever get to the point where we say it might be unethical not to use genome editing for certain applications? That’s a possibility even for germ-line editing, but we’re not there today; we don’t yet have the research to understand how gene editing would work in human situations.

